Graduate student Dan Hickstein (Kapteyn/Murnane group) recently investigated the behavior of electrons ripped from atoms and molecules by intense infrared laser pulses.
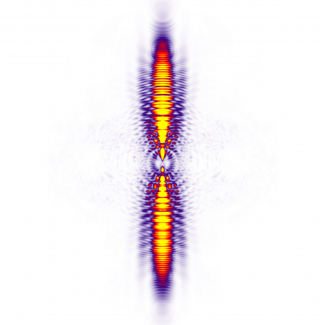
He and his colleagues collected the liberated electrons onto a detector where they formed intricate patterns that looked a lot like giant spiders. An international team of scientists studied these spider-like patterns to learn more about how electrons separate from atoms or molecules. They also tried to discover new information about the structure of the parent atoms or molecules.
The scientists figured out why these intriguing spider patterns are produced. First, the laser field helps an electron to tunnel out of a xenon or argon atom. Second, the laser field accelerates the liberated electron away from the parent atom before hurling it back toward the parent. At this point, one of three things happens to the electron: (1) it can crash back into its parent atom, releasing a photon of x-ray light, (2) it can fly straight past its parent atom, or (3) it can “bounce” off its parent atom, flying off in a new direction.
The researchers realized that, being a quantum mechanical particle, an electron that didn’t reunite with its parent atom could be treated as a wave. The different paths this electron could take could then be treated as waves that will interfere with each other. And, that’s exactly what happened to form the spider patterns. The electron zooming straight by its parent atom is traveling as a plane wave, while the electron that bounces off its parent is traveling as a spherical wave. Beautiful quantum interference patterns between these two possible pathways created the spider structures.
The spider patterns revealed that ionization does not happen abruptly. Some electrons hang out near their parent atoms for multiple laser cycles before bouncing off the atom and escaping. Details in the spider patterns made it possible for the researchers to count the number of times the laser field drove an electron past its parent before it escaped. The patterns also made it possible to make the first experimental measurement of the exact distance an electron traveled to tunnel out of the atom! These exciting results bode well for future experiments to “watch” the complex dance of electrons in atoms in molecules as chemical reactions take place.
Collaborators on this exciting project included former research associates Predrag Ranitovic and Stefan Witte, research associate Ellen Keister, graduate students Craig Hogle and Bosheng Zhang, Fellows Margaret Murnane and Henry Kapteyn as well as colleagues from VU University (the Netherlands), University of Tsukuba (Japan), FOM Institute AMOLF (the Netherlands), and the Max-Born-Institute (Germany).


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.